World
UK Health System Approves New ‘Groundbreaking’ Sickle Cell Disease Treatment
The National Institute for Health and Care Excellence (NICE) announced on Friday that Britain’s National Health Service (NHS) will provide a cutting-edge gene therapy aimed at curing sickle cell disease.
The therapy, developed by Vertex Pharmaceuticals and CRISPR Therapeutics, will cost the state-funded healthcare system approximately £1.65 million ($2.1 million) per course. In 2023, Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) became the first in the world to approve this therapy, which utilizes the revolutionary gene-editing tool CRISPR. NICE, which evaluates the suitability of new medical technologies for use within the NHS, stated that the treatment would be appropriate for around 50 patients annually.
Understanding Sickle Cell Disease
Sickle cell disease is a severe, lifelong condition caused by genetic mutations in the hemoglobin genes. Hemoglobin is the protein in red blood cells responsible for carrying oxygen throughout the body.
The disease can be life-threatening and often leads to recurrent episodes of intense pain when misshapen red blood cells block blood vessels. These abnormal cells become sickle-shaped, stiff, and sticky, unlike the flexible, smooth discs of healthy red blood cells.
Sickle cells have a shorter lifespan than healthy red blood cells and can clump together as they circulate, reducing oxygen delivery to vital organs. This increases the risk of organ damage, stroke, heart failure, and a significantly diminished quality of life.
Promising Results from Clinical Trials
In clinical trials, all patients who received the gene therapy—which modifies a specific gene to enable the body to produce more healthy red blood cells—avoided hospitalizations for at least one year post-treatment, with most remaining hospitalization-free for up to three and a half years. Additional data from ongoing studies are still being analyzed.
NHS Chief Executive Amanda Pritchard described the therapy as “absolutely transformative,” adding that it “could enable patients to live free from the fear of sickle cell crises hanging over them.” The availability of this therapy on the NHS offers hope to patients and signals to the community that sickle cell disease is being taken seriously.
“This is going to be a life-changing moment for many of my patients,” Pritchard said.
How the Gene Therapy Works
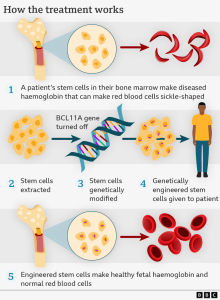
The therapy involves a multi-step process. First, stem cells are extracted from a patient’s bone marrow, where all blood cells are produced. These cells are then sent to a laboratory, where the CRISPR gene-editing tool is used to target and modify a specific gene.
Rather than directly correcting the faulty gene responsible for sickle cell disease, the therapy, known as Casgevy, leverages a natural process that occurs during fetal development. In the womb, babies produce red blood cells containing fetal hemoglobin, a protein that efficiently carries oxygen. After birth, the body switches to producing adult hemoglobin, which is affected by sickle cell disease.
CRISPR works by suppressing the “switch” that triggers the production of adult hemoglobin, allowing the body to continue producing fetal hemoglobin, which is unaffected by the disease.
Before the modified stem cells can be reintroduced, patients must undergo “conditioning” chemotherapy to prepare their bodies to accept the edited cells. Once ready, the modified stem cells are transfused back into the patient, where they multiply and increase the production of stable, functional red blood cells.
Considerations and Challenges
The treatment process is complex and requires careful consideration. It may involve lengthy hospital stays and can have side effects, including headaches and bleeding problems. Currently, the only alternative cure for sickle cell disease is a stem cell transplant, but this option is only viable if a closely matched donor is available. Even then, there is a risk of the transplant being rejected by the recipient’s body.
Availability and Expansion
The gene therapy will be offered at specialist centers in London, Manchester, and Birmingham to patients aged 12 and over who experience recurrent sickle cell crises and are unable to find a suitable stem cell donor.
The therapy has also been approved for another inherited blood disorder, transfusion-dependent beta thalassemia, and is already being administered to patients in countries such as France, Germany, and Italy. Wales is expected to begin offering the treatment within the next few months.
(Reuters, BBC)
Kenya Insights allows guest blogging, if you want to be published on Kenya’s most authoritative and accurate blog, have an expose, news TIPS, story angles, human interest stories, drop us an email on [email protected] or via Telegram
-

 Business1 week ago
Business1 week agoEastleigh Businessman Accused of Sh296 Million Theft, Money Laundering Scandal
-

 Investigations6 days ago
Investigations6 days agoInside Nairobi Firm Used To Launder Millions From Minnesota Sh39 Billion Fraud
-

 Business7 days ago
Business7 days agoMost Safaricom Customers Feel They’re Being Conned By Their Billing System
-

 Business1 week ago
Business1 week agoEXPLOSIVE: BBS Mall Owner Wants Gachagua Reprimanded After Linking Him To Money Laundering, Minnesota Fraud
-

 News6 days ago
News6 days agoUnfit for Office: The Damning Case Against NCA Boss Maurice Akech as Bodies Pile Up
-

 News7 days ago
News7 days agoTax Payers Could Lose Millions in KWS Sh710 Insurance Tender Scam As Rot in The Agency Gets Exposed Further
-

 Sports1 week ago
Sports1 week agoFury as Bettors Demand Probe Into Betika Over Alleged Unpaid Winnings
-
 News7 days ago
News7 days agoPastor James Irungu Collapses After 79 Hours Into 80-Hour Tree-Hugging Challenge, Rushed to Hospital